 7.0 ACID BASE AND SALT
7.0 ACID BASE AND SALT Acid have long been associated with the sour taste of some fruits such as lime and lemon. Their ability to change litmus solution (a vegatable dye) from blue to red is also well-known. There are two classes of acids - Organic and Inorganic acid.
7.1 SOME ORGANIC AND SOURCES * Ethanoic acid - (Source: Vinegar)
* Lactic acid - (Source: Milk)
* Citric acid - (Source: Lime , Lemon)
* Amino acid - (Source: Amino acid)
* Fatty acid - (Source: Fats and oiLs)
* Ascorbic acid - (Source: Oranges)
SOME INORGANIC AND FORMULA * Hydrochloric acid - HCl
* Tetraoxosulphate(vi)acid - H2SO4
* Trioxonitrate(v)acid - HNO3
ACIDS IN SOLUTION An acid is a substance which produces hydrogen ions as the only positive ion when dissolved in water. Acid dissolve in water to produce hydrogen ion H+, as the only positive ions, together with the corresponding negative ions. This process is known as
ionization.
The characteristics properties of an acid solution are due to the presence of these hydrogen ions. This is seen when dry hydrogen chloride gas is dissolved in water and in methyl benzene.
* in water , it forms hydrogen ions and behave like a typical acid
* in methyl benzene, it does not form hydrogen ions and does not show any acidic properties.
THUS: an acid has at least one ionizable hydrogen atom in its molecule.
Strong acids ionize completely in water to give hydrogen ions and anions. The concentration of hydrogen ions is very high in such acid solutions. following are examples of strong acid.
Hydrochloric acid - Hcl --> H+ +Cl-
Trioxonitrate(v) acid - HNO3 ---> H+ + NO3-
Tetraoxosulphate(vi) acid - H2SO4 ---> 2H+ + SO4^2-
Weak acid are only partially ionized in water. such acid solution have a low concentration of hydrogen ions. For example ethanoic acid has only 0.4% ionization in water, i.e only four out of every thousand acid molecule will ionize in water. Other examples are weak acids include trioxocarbonate(iv)acid, tetraoxophosphate(v)acid and most organic acid.
7.2 BASICITY OF AN ACID The basicity of an acid is the number of replaceable hydrogen ions H+, in one molecule of the acid. All acid in an aqueous solution yield hydrogen ions which can be replaced by metallic ions. The number of such hydrogen ions present in one molecule of an acid is known as basicity of this acid
BASICITY OF SOME COMMON SALT HCL - H+ + Cl- (has 1 or monobasic basicity)
H2SO4 - 2H+ + SO4 (has 2 or dibasic basicity)
H3PO4 - 3H+ + PO4 (has 3 or tribasic basicity)
Not all the hydrogen atoms in a molecule of an acid are replaceable by metal.
Ethanoic acid, CH3COOH, For instance has four hydrogen atom per molecule of acid, but only one of these is replaceable by metal.
Ethanoic acid is
mononasic7.3 PHYSICAL PROPERTIES OF ACID 1. Dilute acids have a sour taste. The sour taste of unripe fruit, vinger and rancid milk is due to the presence of acid in them.
2. Acid turns
blue litmus paper red.
3. The concentarted forms of strong acids like hydrochloric acid, trioxonitrate(v)acid and tetraoxosulphate(vi) acid are
corrosive7.4 CHEMICAL PROPERTIES OF ACID A.
Reaction with Metals Acid react with some metals like Zinc, iron and Magnesium to liberate hydrogen gas. Such reactions are due to the displacement of the hydrogen ions in the acids by the metals. Dilute trioxonitrate(v) acid, however is an exception of this rule.
2HCl(aq) + Zn(s) ---> ZnCl2(aq) + H2(g)
B.
Reaction with Bases Acid react with insoluble bases and alkalis to form salts and water as the only products. Such a reaction is known as
Neutralization 2HCl(aq) + CaO(s) ---> CaCl2(aq) + H2O(l)
C. Reaction with Trioxocarbonate(iv)
Acid react with trioxocarbonate(iv) to librate carbon(iv) oxide.
2HCl(aq) + Na2CO3(aq) ---> 2NaCl(aq) + H2O(l) + CO2(g)
NOTE: The three chemical properties listed above are characteristics properties of acids.
7.5 PREPARATION OF ACIDS An outline of the general methods for the preparation of acid is given here;
1. Dissolving an acid anhydride in water
* Carbon(iv)oxide dissolves in water to form a weak acid, trioxocarbonate(iv) acid.
CO2(g) + H2O(l) ---> H2CO3(aq)
* Sulphur(iv) oxide dissolves to form trioxosulphate (iv) acid in water.
SO2(g) + H2O(l) ---> H2SO3(aq)
* Sulphur(vi) oxide dissolves to form trioxosulphate (iv) acid in water.
SO3(g) + H2O(l) ---> H2SO4(aq)
2. Combination of constituent elements
* Burning hydrogen in chlorine, in the presence of activated charcoal as the catalyst, yields hydrogen chloride gas which dissolves readily in water to give hydrochloric acid.
H2(g) + Cl2(g) ---
(activated charcoal)---> 2HCl(g)
* Heating hydrogen gas and bromine vapor, in the presence of platinum as the catalyst, produces hydrogen bromide gas, which dissolves readily in water to form hydrobromic acid
H2(g) + Br2(g) ---
platinum---> 2HBr(g)
3. Using a strong acid to displace a weak acid or a volatile acid from its salt.
A strong acid may be used to displace a more volatile acid or a weaker acid from its salt.
* Concentrated tetraoxosulphate(vi)acid displaces the more volatile trioxonitrate(v)acid from a trioxonitrate(v) salt. The salt e.g Sodium trioxonitrate(v), must be excess.
* A Chloride yield the volatile hydrogen chloride gas when heated with concentrated tetraoxosulphate(vi)acid. The volatile hydrogen chloride dissolves readily in water to give hydrochloric acid.
* Dilute hydrochloric acid which is a strong acid displaces the weak trioxocarbonate(iv)acid from trioxocarbonate(iv). The trioxocarbonate acid break down to involve carbon(iv)oxide.
7.6 USES OF ACID Acid are extremely useful chemicals which are used in many industries to make other customer chemicals such as "fertilizer , detergent , drugs". They are used in industrial process as drying agent, oxidizing agent and catalyst.
7.7 BASE AND ALKALIS The term base was originally used to describe substance that turned red litmus blue and neutralized the properties of acids in aqueous solutions. Most oxides and hydroxides of metals are bases. Common examples of basic oxides are sodium oxide Na2O, Potassium oxide K2O, Magnesium oxide Mg2O. They are formed when these metals burn in air or oxygen.
4Na(s) + O2(g) ---> 2Na2O(s)
Most of these metallic oxides are insoluble in water. some, however dissolve in water to form hydroxides. for example;
K2O(s) + H2O(l) ---> 2KOH(aq)
A soluble hydroxide is known as an alkali. many basic hydroxide like copper hydroxide, Cu(OH)2 water. The few soluble hydroxides like the hydroxide of sodium , potassium and calcium are economically important.
An alkali is a basic hydroixde which is soluble in water. Like acids, alkalis may be strong or weak. Sodium and Potassium hydroixdes are strong alkalis that ionize completely in aqueous solutions to produce negatively charged hydroxide ions, OH-, and positively charged metallic ions. Calcium oxide react with water to give a hydroxide which is only slightly soluble in water. A calcium hydroixe solution produces relatively few ions. it is a weak alkali.
CaO(s) + H2O(l) ---> CaOH2(aq)
Another common alkali is aqueous ammonia, formed when ammonia is bubbled through water.
NH3(g) + H2O(l) ---> NH3H2O(aq)
Aqueous ammonia will ionize to a limited extent in solution to liberate ammonium ions, NH4+, and hydroxide ions, OH-. Hence, aqueous ammonia is also a weak alkali.
7.8 NEUTRALIZATION
This is a process in which an acid reacts completely with an appropriate amount of an alkali (or any other base) to produce a salt and water only.
BASE A base is a substance which will neutralize an acid to yield salt and water only.
PHYSICAL PROPERTIES OF ALKALIS 1. Alkalis have a bitter taste
2. Alkalis are soapy to the touch
3. Alkalis turns red litmus blue
4. Concentrated form of two caustic alkalis, sodium hydroxide and potassium hydroxide are corrosive.
USES OF ALKALIS Alkalis are important substance used in industries concerned with the manufacture of glass, soap, paper and rayon. Some alkalis are also used to soften hard water.
7.9 MEASUREMENT OF ACIDITY & ALKALINITY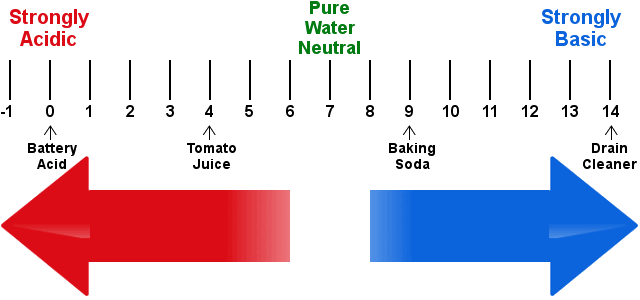
pH is the acidity and alkalinity of a solution. The pH scale with unit ranging from 0 to 14 measures the acidity and alkalinity of substance, A neutral solution has a pH of 7. Acidic solutions have pH value less than 7, while alkaline solution have pH value greater than 7. Indicator are compound which change colour in accordance with the pH of the medium.
COLOUR CHANGES OF SOME INDICATOR * Methyl orange - pH range of 3.1 - 4.6
(color: Orange , Acidic: Red , Alkaline: Yellow)
* Litmus - pH range of 5.0 - 8.0
(color: Purple, Acidic: Red, Alkaline: Blue)
* Phenolphthalein - pH range of 8.3 - 10.0
(color: Pale pink, Acidic: Colourless, Alkaline: Pink)
Use of universal indicator to compare the pH of 1.0M solutions of the following;
* Strong acid e.g hydrochloric acid
* Weak acid e.g ethanoic acid
* Strong alkali e.g sodium hydroxide solution
* Weak alkali e.g aqeous ammonia
* Pure distilled water
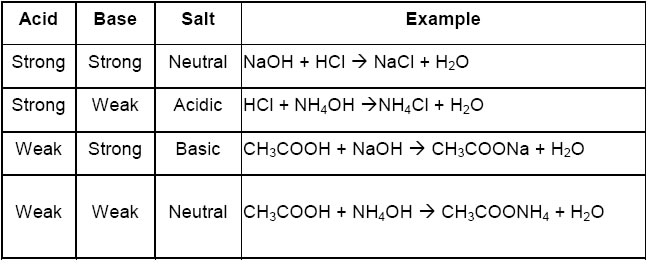
8.0 SALT A salt is a compound formed when all part of the ionizable hydrogen of an acid is replaced by metallic or ammonium ions.
8.1 TYPE OF SALT 1.
Normal Salts: are formed when all the replaceable hydrogen ions in the acid have been completely replaced by metallic ions. Normal salt are neutral to litmus
HCl(aq) + NaOH(aq) ----> NaCl(aq) + H2O(l)
H2SO4(aq) + ZnO(s) ----> ZnSO4(aq) + H2O(l)
2.
Acid salts: are formed when all the replaceable hydrogen ions in an acid are partially replaced by a metal. They turn blue litmus red.
H2SO4(aq) + KOH(aq) ----> KHSO4(aq) + H2O(l)
KHSO4(aq) + KOH(aq) ----> K2SO4(aq) + H2O(l)
3.
Basic salts: contains the hydroxide ion, they occurs when there is an insufficient supply of acid which is needed for the complete neutralization of the base. basic salts turns red litmus blue and will react with excess acid to form normal salt and water.
Zn(OH)Cl(aq) + HCl(aq) ---> ZnCl2(aq) + H2O(l)
4.
Double salts: are salt which ionize to produce three different types of ions in solution. usually two of these are positively charged while other is negatively charged.
5.
Complex salts: contains complex ions i.e ions consisting of a charged group of atoms
Na2Zn(OH)4(aq) >>>> reversible <<<< 2Na+(aq) + [Zn(OH)4]^2-
8.2 USES OF SALT Salt are used in the manufacture of many industrial agricultural and consumer substance like chlorine gas, fertilizer and laxatives. They are also used as food preservatives , drying agent.
Efflorescent: Some crystalline salt will lose part of their water of crystallization when they are exposed to atmosphere to form a lower dehydrate or the anhydrous salt.
Deliquescent: Some compound tends to absorb a large amount of water on exposure to the atmosphere so that they eventually turn into solution.
Hygroscopy: these also absorb moisture on exposure to the atmosphere. if they are solid, they will not form solution but merely became sticky or moist.
Drying agent: these are substance that have a strong affinity for moisture or water. they may be either hygroscopic or deliquescent. they are usually used to dry gas in laboratory.
8.3 PREPARATION OF SALT A. Soluble salt may be prepared by * action of dilute acid on a metal
* neutralization of an alkali by acid
* action of dilute acid on an insoluble base
* action on a dilut acid on a trioxocarbonate(iv)
Salt are then recovered from solution by evaporation or crystallization
B. Insoluble salt are formed by * double decomposition
* direct combination of two element
N.B: All acid, base and salt are electrolytes as they tends to exist in solution as ions, which are the carriers of electricity.
[1] What is an acid ?
[2] What is basicity of an acid ?
[3] What is a base ?
[4] Give 3 physical properties of an acid
[5] What is pH ?
[6] What is neutralization ?
[7] What is the uses of an acid ?
[8] How can we prepare an acid ?
[9] What is an alkali ?
[10] Give 3 physical properties of alkalis ?
[11] What is the important of pH to our body ?
[12] Define a salt
[13] Give all the types of salt you know
[14] What is a Normal salt, Acidic salt, Basic salt ?
[15] What is the uses of salt ?
[16] How can we prepare a salt ?
[17] Define the following
i. Efflorescence
ii. Deliquescent
iii. Drying agent
iv. Hygroscopy





