 4.O ATOMIC STRUCTURE , CHEMICAL COMBINATION
4.O ATOMIC STRUCTURE , CHEMICAL COMBINATION According to
Dalton's Atomic Theory, Atoms were indestructible solid particles. However, experiment involving electrolysis indidcate that certain compound contains charged particles called
ion. The formation of ions could not be explained by Dalton's Atomic Theory. As a result of this and other observation, scientist had to modify their views.
4.1 (SUMMARY) ATOMIC THEORY In 1808, John Dalton proposed the
Atomic Theory which can be summarized below
* All element are made up of small, indivisible particles called
atoms.
* Atoms can neither be created nor destroyed.
* Atoms of the same element are alike in every aspect, and differ from atoms of all other element.
* When atoms conbine with other atom, they do so in simple ratio.
* All chemical changes result from the combination or the seperation of atoms.
4.2 SUB-PARTICLES OF ATOM The atom is made up of three main sub-particles (I)
Proton (II)
Electron (III)
Neutron Particle (
Proton) has (
1 Mass unit), (
+1 Charges) and (
p symbol).
Particle (
Electron) has (
1/1840 Mass unit), (
-1 Charges) and (
e symbol).
Particle (
Neutron) has (
1 Mass unit), (
No charges) and (
n symbol).
4.3 BOHR'S MODEL OF THE ATOM In 1913, Niels Bohr used the Quantum Theory ideas developed by Planck and Einstein to account for the spectrum of hydrogen. He made the following assumption;
* Rutherford's model of the atom is basically correct.
* Each spectral line is caused by an electron.
* Electron can exist only in circular orbits.
* An electron emits energy in the form of radiation when it moves from a higher to a lower permitted orbit - This produces a line in the atomic emission spectrum.
4.4 WAVES MECHANICS MODEL The Waves Mechanics Model of the atom does not restrict electrons to definite regions around the nucleus as the Bohr's model does. Instead, it makes the electron elusive and indicates a region around the nucleus called an orbit where there is a possibility of finding an electron with a certain given amount of energy.
The energy of an electron is characterized by four quantum numbers. They are the principal quantum number n, the subsidiary quantum number 1, the magnetic quantum number m, and the spin quantum number s.
The arrangememnt of electron around the nucleus of an atom is determined by four quantum number of each electron in the atom.
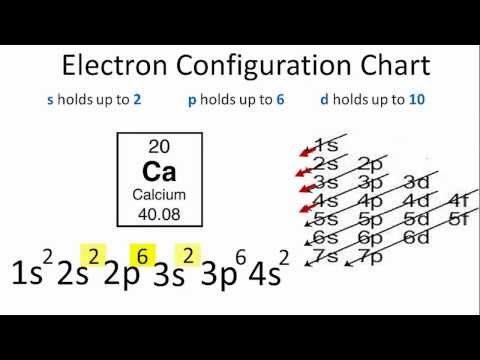
[1] Hydrogen = 1s1
[2] Helium = 1s2
[3] Lithium = 1s2 2s1
[4] Berrylium = 1s2 2s2
[5] Boron = 1s2 2s2 2p1
[20] Calcium = 1s2 2s2 2p6 362 3p6 4s2

The atomic number A of an element is the number of proton in one atom of that element. The atomic number is a basic property of an element.
The mass number z of an element is the sum of proton and neutron in its atom.
4.5 ISOTOPES Isotopy is whereby atoms of an element exhibit different mass number but have same atomic number. Isotopes of an element are represented by the symbol of the element with the mass and atomic numbers.
When element are arranged in ascending order of atomic number, element with similar chemical properties occurs at regular period. This is the basis for the arrangement of element in the priodic table.
The chemical stability of rare gases is due to the duplet and octet electronic configuration in the outermost shells of their atoms. Other element have a tendency to archieve these stable configuraions through chemical combination.
There are two main type of Chemical combinations. [1]
Electrovalent Combination: There is transfer of electron(s) from one atom to another resulting in an electrovalent bond between ions.
[2]
Covalent Combination: Electron pairs are shared between two atoms resulting in an ordinary covalent bond (if each atom contributes one electron), or a coordinate covalent bond (if one atom donate both electron).
Double and Triple covalent bond results when two and three pairs of electron respectively are shared between the bonding atoms.
Electrovalent or Ionic compound are composed of ions arranged in an orderly pattern to form crystal lactics. They are hard, brittle, solid with a high melting point. in the molten or aqueous state, they conduct electricity. They are soluble in polar solvent like water.
Simple covalent compound are usually small molecule with a definite shape. They are often gases or volatile liquid. if they are solid, they have meleting point. Covalent compounds are nonelectrolyte and are usually soluble in non-polar solvents.
Van der waals forces and the hydrogen bond are weak intermolecular bonds.
4.6 ELECTRONIC CONFIGURATION OF RARE GAS
Helium (Atomic number 2), Have only 2 Shell (K)
Neon (Atomic Number 10), Have 2,8 Shell (K L)
Argon (Atomic number 18), Have 2,8,8 Shell (K L M)
Calcium (Atomic number 20), Have 2,8,8,2 Shell (K L M N)

[1] What re the 3 fundamental unit of all matter ?
[2] Give their relative masses and charge to QUE 1
[3] From QUE 1 Describe their relative positions to one another in an atom.
[4] What did Thomas Young, Max Planck And Albert Einstein contribute towards the nature of light?
[5] Explain simply how the line in a hydrogen spectrum are obtained.
[6] State Dalton's Theory about the atom.
[7] Outline in details the modern atomic concept & show how it modifies Dalton's theory.
[8] Give the electronic configuration of (I) Potassium (II) Magnesium
[9] The electronic configuration of potassium is 2, 8, 8, 2. Explain this
[10] Shows why potassium ia a very reactive metal from QUE 9.
[11] What are isotopes ?
[12] Name two element that exhibit isotopy and give their respective isotopes.
[13] Write briefly on the following (I) Electrovalent (II) Covalent Combination.
[14] Explain why relative atomic mass of chlorine is 35.5
[15] How many proton are there in sodium
[16] How many neutron are there in sodim
[17] How many electron are there in sodium
[18] Why does solid sodium chloride not conduct electricity?
[19] Explain why Rare gases are stable
[20] An element belong to a period in periodic table because of what?
Visit
www.spyloadedng.com/cbt/ for Quiz







